As a contract design and manufacturing organization (CDMO), we work closely with our customers to deliver proven, highly engineered products that help millions of people live healthier, more productive lives. Autoinjectors and pens for delivering insulin, GLP1, epinephrine and other life-saving drug treatments. Advanced, wearable injection pumps. Point-of-care and at home diagnostic tests. Electrophysiology technology. Continuous glucose monitoring systems. And more. Explore how we can help you.
The largest pharmaceutical, medtech and in vitro diagnostic companies in the world count on Phillips-Medisize to bring their breakthrough ideas and innovative branded products to market – with confidence.
A 3rd prestigious award for Ascendia Pharma and Phillips-Medisize for the SKYTROFA ® Auto-Injector - The first-of-its-kind product that enables convenient and efficient preparation and delivery of SKYTROFA—a vital growth hormone treatment for children.

Phillips-Medisize Teams with GlucoModicum on Innovative Device
See how seamless collaboration and human-centered design create a first-of-its-kind, proprietary, needle-free continuous glucose monitor (CGM) that integrates sophisticated technology with advanced biosensors and algorithms.

Award Recognition: Red Dot Design Award
In collaboration with Ascendis Pharma, Phillips-Medisize was recently recognized with a third prestigious award for the SKYTROFA Auto-Injector.

Aria Smart Autoinjector
Our Aria smart autoinjector is a powerful, flexible, platform, designed for ease of use with a compact form and aligned to key emerging global trends.
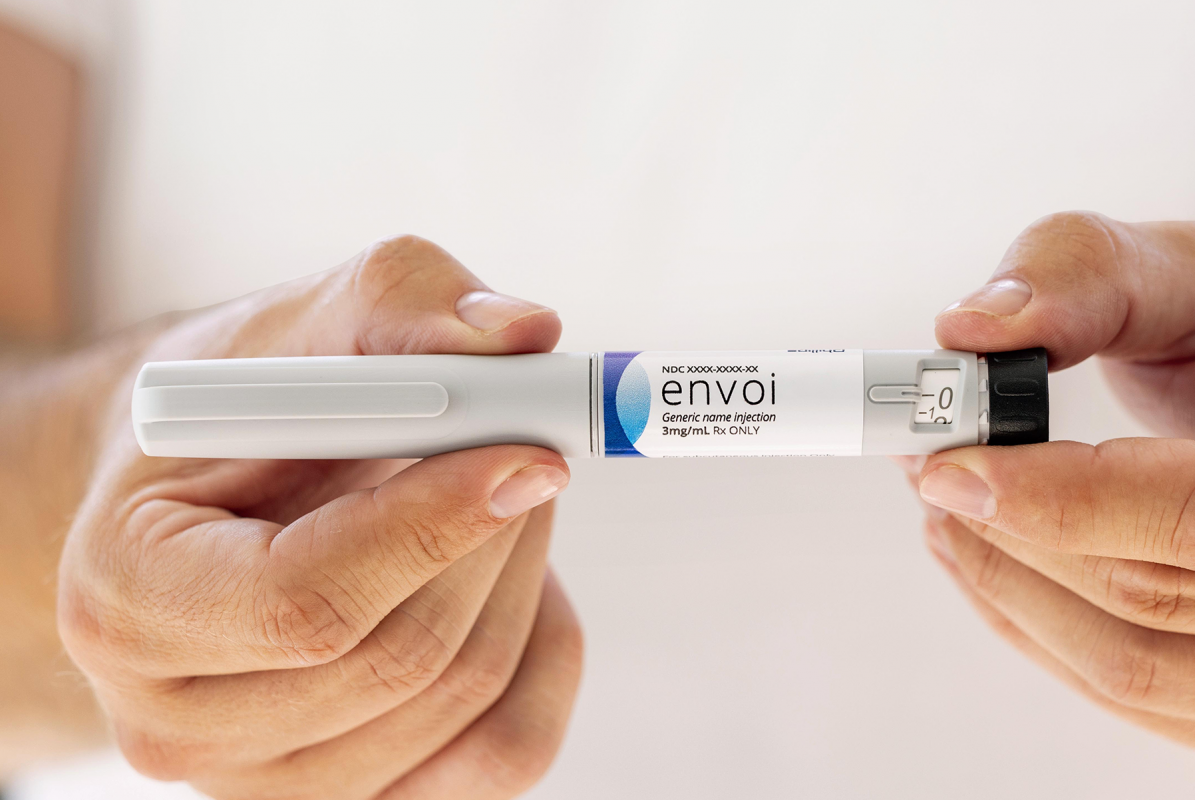
Envoi Pen Injector
A familiar, state-of-the-art design in a market-ready, scalable platform for fast, high-volume rollout of novel or generic drug treatments.
Blending Innovative Science and Advanced Manufacturing
Game-changing product, transparent teamwork and world-class manufacturing have amplified patient screenings for early cancer detection. Thanks to a powerful collaboration between Phillips-Medisize and Exact Sciences.

Explore the New Phillips-Medisize Poland Campus
Discover how the initial 23,000 square meters of manufacturing space in Katowice will serve as a strategically centralized European location—offering expansive production capabilities, facilitating timely product delivery and creating hundreds of skilled new jobs.

Make Informed Choices for Agile Product Development
Designing, producing and delivering a commercially available medical product is incredibly challenging—especially when that product is a drug delivery device. Request our whitepaper for actionable insights that support your market success.

Explore Our Award-Winning Device Design
-Read the article published by LifeSciencesIntelligence to learn why Phillips-Medisize recently received its third industry award for a uniquely designed drug delivery device. The auto-injector is a dual-chamber, automatic, reusable device for pediatric patients with growth hormone deficiency.

About Us
Bringing Possibilities to Life
Trusted for nearly 60 years, Phillips-Medisize, a Molex company, is a global leader in front-end design, development and manufacturing solutions for highly regulated industries — pharma, in vitro diagnostic, med tech, consumer, automotive and defense.

Careers
Join a team of industry-leading innovators
Phillips-Medisize is comprised of a global network of professionals spanning across 36 locations in 11 countries. We build products to enhance the lives of all our customers around the world in pharmaceutical, in vitro diagnostic, medical technology, consumer, automotive and defense fields. The work we do matters.
Contact Us

